Schedule a Call
Ready to Upgrade Your Process Operations?
Learn how corrosion-resistant metals perform in harsh industrial environments, which alloys fit conditions, and how material choice affects service life costs.


Corrosion rarely announces itself early. It shows up after installation, once equipment is in service, when replacement costs, downtime, and safety risks are hardest to absorb. For manufacturing engineers, the challenge is familiar. Materials meet specification on paper, yet degrade faster than expected in real operating environments.
This blog breaks down the most effective corrosion-resistant metals used in industrial applications today. It explains how different alloys respond to specific environments and how material choice influences maintenance effort and long-term performance.
Corrosion reduces load-bearing capacity over time, increasing the risk of cracking, leakage, and premature failure in pressure-bearing or structural components.
In critical applications, corrosion resistance is less about durability on paper and more about reliability in the field.
Once the impact is clear, the next step is understanding how corrosion resistance is engineered at the material level.
Corrosion resistance is engineered through material behavior, surface chemistry, and processing choices that limit how metals interact with their environment.
Together, these mechanisms determine how well a metal resists degradation in a real industrial environment.
Also Read: Aircraft Alloys: Properties, Types, and Aerospace Engineering Insights
These mechanisms differ across alloy families, which is why material selection depends heavily on the operating environment.
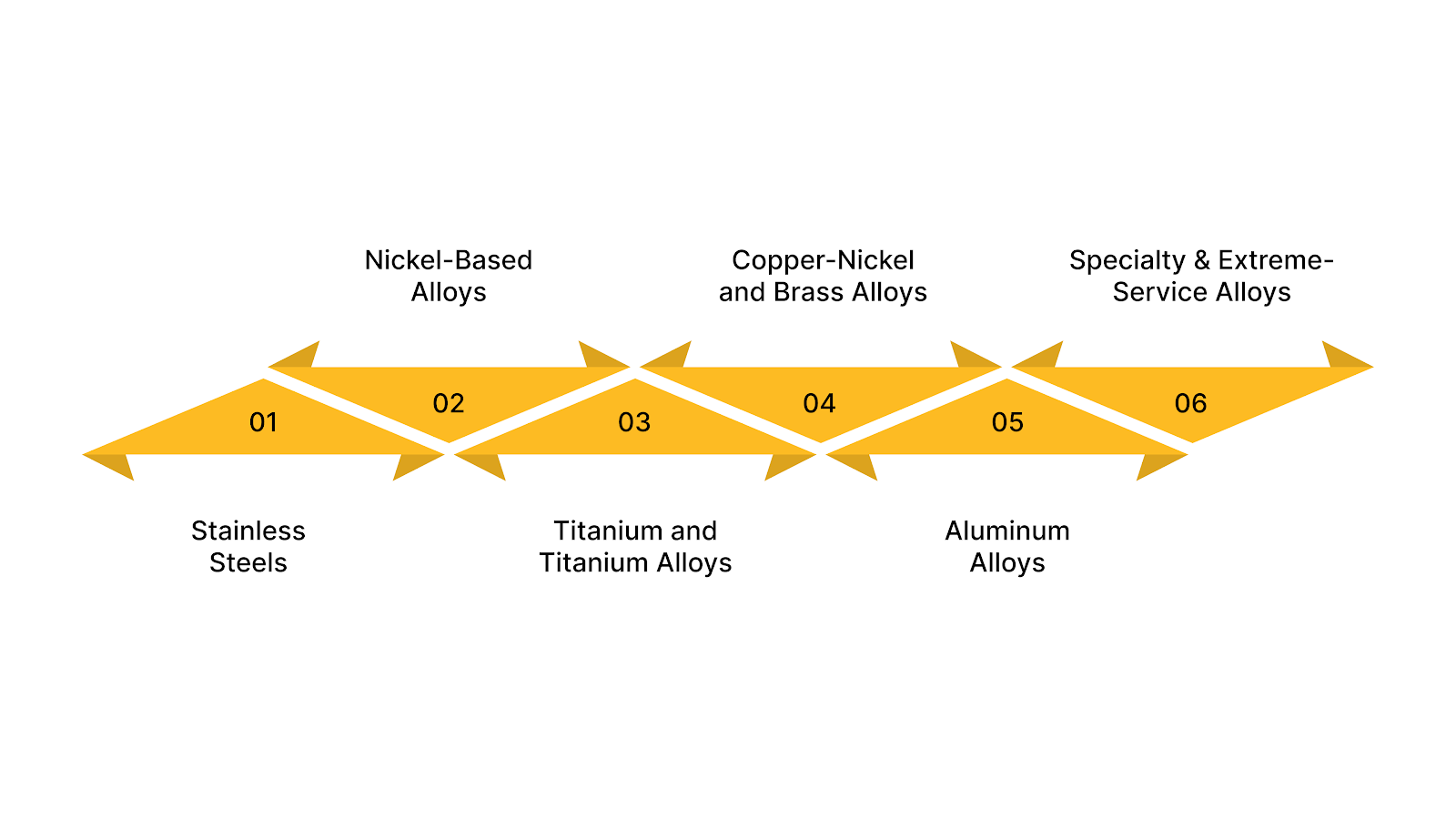
Corrosion resistance depends on chemistry, temperature, stress, and exposure time. The metals below are commonly selected because their corrosion mechanisms are well understood and predictable when matched correctly to the environment.
Stainless steels resist corrosion through chromium-driven passive oxide formation. Performance varies significantly by grade and environment.
Best suited for: Food and pharmaceutical equipment, chemical processing systems, marine hardware, pressure vessels, heat exchangers, and desalination plants.
Nickel alloys are selected when corrosion severity, temperature, or mixed chemical exposure exceed the limits of stainless steel.
Best suited for: Chemical reactors, offshore systems, scrubbers, turbines, pollution control equipment, nuclear, and petrochemical applications.
Titanium’s corrosion resistance stems from an extremely stable oxide layer that reforms instantly upon damage.
Best suited for: Heat exchangers, desalination plants, marine systems, aerospace components, and chemical processing equipment.
Copper alloys resist corrosion through protective surface films and natural biofouling resistance.
Best suited for: Marine piping, cooling systems, condensers, seawater handling equipment.
Aluminum relies on a natural oxide layer for corrosion protection, which can be enhanced through anodizing or coatings.
Best suited for: Aerospace structures, storage tanks, transportation systems, and architectural components.
These alloys are chosen when corrosion occurs alongside heat, pressure, or mechanical wear.
Best suited for: Chemical processing plants, nuclear systems, high-wear industrial components, severe service environments.
Corrosion resistance is not about choosing the “best” metal. It is about selecting the alloy whose corrosion behavior stays stable under your exact operating conditions.
When corrosion margins are tight and compliance is non-negotiable, Aero-Vac Alloys & Forge supports procurement teams with certified stainless steels, nickel alloys, titanium, and specialty metals, delivered with full traceability and value-added processing. From material selection to near-net-shape delivery, we help keep mission-critical programs moving without surprises.
Even within proven alloy categories, performance varies, making selection criteria just as important as the alloy name itself.
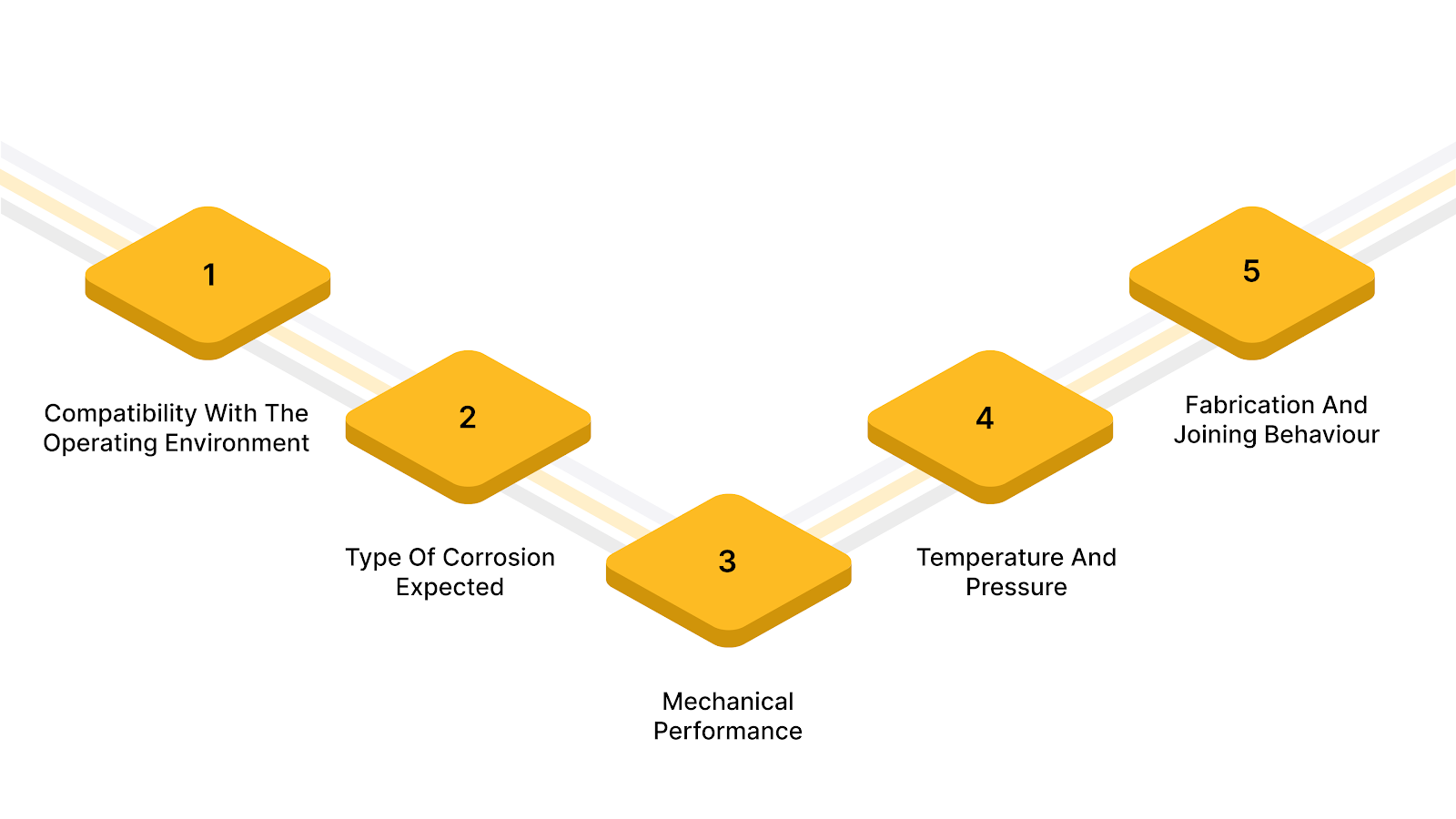
Selecting a corrosion-resistant alloy requires aligning material chemistry, service environment, and fabrication constraints from the start. Here’s a breakdown:
Corrosion resistance depends on how the alloy reacts with specific chemicals, salts, moisture, or process fluids present in service. Performance against one corrosive medium does not translate automatically to another.
Uniform corrosion, pitting, crevice corrosion, and galvanic corrosion place very different demands on alloy chemistry. Localized corrosion is often more dangerous than general thinning due to the sudden loss of section.
The alloy must retain strength, ductility, and fatigue resistance under corrosive conditions. Corrosion-assisted cracking becomes a concern in high-stress environments.
High temperatures and pressure cycles can accelerate corrosion mechanisms and degrade protective oxide layers. Thermal cycling often reveals weaknesses that static testing does not.
Weldability and heat-affected zone performance affect corrosion resistance in real assemblies. Poor fabrication choices can negate the inherent corrosion resistance of the base alloy.
Selecting the right corrosion-resistant alloy is about controlling uncertainty, so material behaviour remains predictable throughout service and under continuous monitoring.
Also Read: Alloy Forge Explained: Proven Techniques & Applications in 2026
Selecting the right alloy is only part of the equation. Consistent supply, processing control, and documentation determine how that choice performs in service.
Corrosion resistance depends on selecting alloys proven for the environment, controlling melt quality, processing, and documentation through delivery. Aero-Vac supports that end-to-end.
When corrosion resistance cannot rely on assumptions, Aero-Vac helps teams lock in material performance before parts enter service.
Corrosion resistance is achieved by selecting alloys proven for the environment and controlling melt quality, processing discipline, and documentation from mill to final delivery. When material behavior is consistent, inspection results become clearer, and engineering teams spend less time questioning whether corrosion signals reflect real degradation or inherited variability.
Aero-Vac Alloys & Forge supports corrosion-critical programs by supplying vacuum-melted, fully traceable stainless steels, nickel-based alloys, and titanium grades, combined with in-house forging, heat treating, and precision cutting.
Contact us to discuss selecting corrosion-resistant alloys, melt quality, and processing controls tailored to your service environment and compliance requirements.
1. What makes a metal truly corrosion-resistant in industrial environments?
Corrosion resistance depends on alloy chemistry, microstructure, and surface condition. Elements like chromium, nickel, molybdenum, and titanium form protective layers that slow degradation in aggressive environments.
2. How do stainless steels differ in corrosion performance?
Not all stainless steels perform the same. Grades like 316 and 347 resist chlorides and high temperatures better than 304, while PH grades balance corrosion resistance with high strength.
3. When are nickel-based alloys preferred over stainless steel?
Nickel-based alloys such as 625, 718, and X-750 are used when corrosion combines with high temperature, pressure, or cyclic loading. Stainless steels reach limits sooner in these conditions.
4. Why does melt practice matter for corrosion resistance?
Vacuum-melted alloys contain fewer inclusions and tighter chemistry control. This reduces pitting initiation sites and improves resistance to stress corrosion cracking in long-term service.
5. How does processing affect corrosion performance after material selection?
Forging method, heat treatment, and machining influence residual stress and surface integrity. Near-net-shape processing and controlled heat treatment help preserve corrosion resistance in service.


